When talking about the revolutionary proteomics work we hope to enable with the NautilusTM Proteome Analysis Platform, we think about two general research modalities:
- Broadscale proteomics
- Targeted proteoform studies
Broadscale proteomics, sometimes also called discovery proteomics, or untargeted proteomics, is an approach that provides researchers with a snapshot of protein identities and abundances across the entire proteome. Broadscale proteomics is especially useful when studying new samples, or for getting a high-level understanding of the various biological processes happening inside a cell or organism.
Watch this animation to discover how we do broadscale proteomics on the Nautilus Platform
Targeted proteoform studies, on the other hand, zoom in on a particular protein or set of proteins and quantify their many possible modifications and variations (their proteoforms). Both targeted and untargeted proteomics are designed to generate novel and impactful biological insights and both are necessary to ensure the proteomics revolution bears fruit.
In this blog post, we cover technical aspects of broadscale proteomics on the Nautilus Proteome Analysis Platform. We also describe the overarching problems in proteomics expected to be solved by the platform and discuss a few ways broadscale proteomics may be applied in future research efforts. In a companion blog post, we dive into targeted proteoform studies.
Broadscale proteomics on the NautilusTM Platform
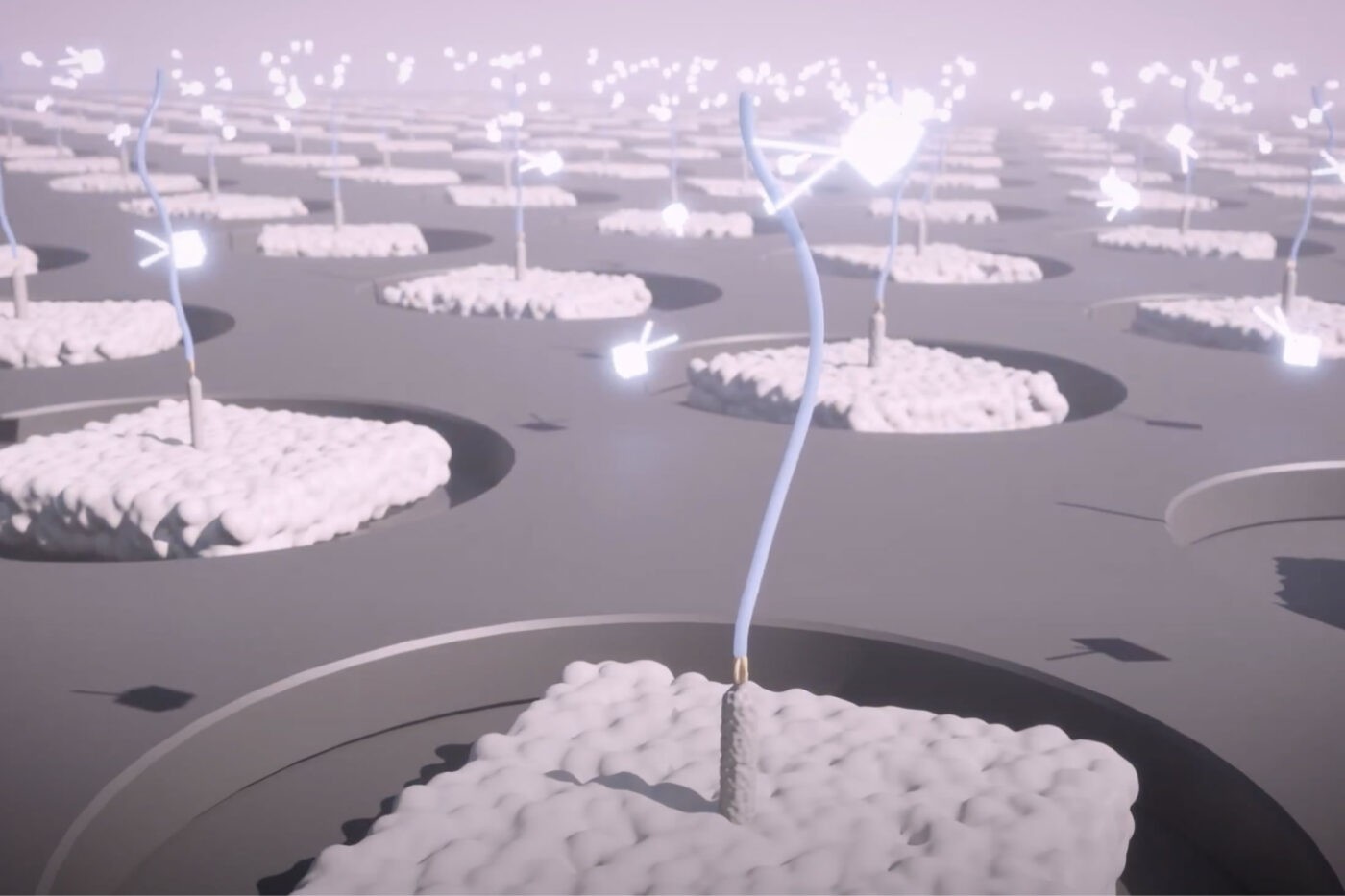
We aim to achieve broadscale proteomics using “Iterative Mapping.” We cover this method in detail in our preprint, but in brief, it is designed to enable our platform to identify and quantify more than 95% of the proteome in a comprehensive, sensitive, reproducible, and rapid way. It is expected that this untargeted proteomics methodology will cover the wide dynamic range of the proteome with an accessible, integrated workflow from sample to insight.
Applying Iterative Mapping to proteins, the NautilusTM Proteome Analysis Platform repeatedly interrogates billions of single, intact protein molecules on our massive, nanofabricated protein arrays using multi-affinity probes that bind to short protein sequences (~3 amino acids). Our machine learning-powered algorithms use the observed binding patterns to identify each protein at the single-molecule level, and identifications are summed to provide protein counts. This method thus provides quantitative maps of the proteome.
Although an individual multi-affinity probe cannot determine protein identity since the short sequences are present in many proteins, binding patterns unique to each protein emerge through iterative cycles of interrogation. This enables detection of >95% of the proteome with roughly 300 probes.
In addition, our nano-fabricated arrays can accommodate up 10 10 billion proteins. This should enable us to measure proteins varying in abundance over 9 orders of magnitude with more than 95% proteome coverage across species and sample types.
Aiming to solve major issues in broadscale proteomics
Older proteomics technologies like mass spectrometry are routinely used for untargeted proteomic analysis but rarely cover the full dynamic range of the proteome. Thus, signals from high abundance proteins often drown out those from low abundance proteins and likely mask essential biological insights. These technologies also infer protein identity from pools of protein fragments (peptides) instead of full-length proteins. This makes it difficult to generate reproducible, quantitative results.
Other protein analysis techniques may alternatively rely upon the creation of highly specific affinity reagents for each and every protein in the proteome. This is very difficult to scale to the 20,000+ proteins in the human proteome for true untargeted proteomic studies and makes it challenging to do cross-species comparisons in translational studies.
On the Nautilus Proteomic Analysis Platform, multi-affinity probes, single-molecule, intact protein analysis, and robust machine learning-powered protein identification are designed to make it easier to capture the breadth and depth of the proteome.
Applications of broadscale proteomics
To understand how such broadscale proteomic analyses could be used, it’s informative to look at previous proteomics studies. We hope to enable scientists to vastly expand on similar work in the future, and recent publications represent just the tip of the iceberg in terms of what researchers can reveal with comprehensive broadscale proteomics.
For example, researchers working with Nautilus Scientific Advisory Board member Professor Ruedi Aebersold used a multiomics approach to discover biological differences across HeLa cell lines. These rapidly growing cells are commonly used in many studies of human cell biology, but acquire important physiological differences as they are propagated across labs. Broadscale proteomics revealed that differences in protein levels across HeLa cell lines lead to vast differences in cellular morphology, growth rates, response to micro RNA transfection, and susceptibility to Salmonella infection. Here, broadscale proteomics revealed differences in a common research tool that could have vast impacts on how that tool is used in the future. These analyses may lead to more reproducible results across many future research efforts using HeLa cells.
While this study focused on the basic biology of an important research tool, broadscale proteomics has many, many applications in applied biology including:
In addition, our recent white papers dive into applications of proteomics in basic research, precision medicine, and novel cancer treatments. The potential applications of accessible broadscale proteomics are nothing short of inspiring and we’re only scratching the surface.
An exciting future for broadscale proteomics
Broadscale proteomics studies reveal a wealth of data that can lead to many further experiments and insights. These can truly change the way researchers think about and even conduct experiments. We are excitedly working to make such studies far more accessible, robust, and comprehensive with our novel platform. We cannot wait for the treasure trove of insights we hope to enable.
MORE ARTICLES