
Unlocking the complexity of proteins to fuel precision medicine (Part 1)

Tyler Ford
April 13, 2023
When the first draft of the human genome was completed in the early 2000s, many hoped the ensuing genomics revolution would usher in a new era in drug development. Researchers would use their understanding of the genome to create personalized “precision medicines” that target diseases at their root genetic causes and have a high likelihood of clinical success. However, this has not been possible for most diseases, and only around 13% of all drugs entering clinical trials make it to market (Wong et al 2019). Billions of dollars still go into developing drugs that ultimately fail (Wouters et al 2020). Here we describe how proteomics can radically improve the drug development process.
The need for a proteomics revolution
Instead of being the hoped-for panacea, the genomics revolution has highlighted just how complex biology is. Genes generally encode proteins, the molecular machines that carry out most cellular processes. A cell’s full set of genes, its “genome,” is generally static. By contrast, a cell’s full set of proteins, its “proteome,” is highly dynamic. Proteins move around within cells, and the composition of the proteome depends on a cell’s position in the body, age, stimuli, and more. We cannot analyze the proteome using genomics, but the vast majority of approved drugs target proteins (Santos et al 2017). Thus, being blind to the proteome and its complexity makes it difficult to develop effective drugs.
To accelerate drug development, we need to unlock the complexity of proteins and harness the power of proteomics.
Unlike its DNA-based counterpart (the genome), if you start talking to people about the proteome you’ll get blank stares. Most people probably only think of proteins as parts of their diets – mysterious things listed on cereal boxes. But, if the genome is the blueprint for your cells, the proteome is the live tour of your home, complete with your children running from place to place and painting the walls. It provides us with much more information than the genome and changes from day to day, hour to hour, minute to minute.
Given that the proteome is composed of millions of protein isoforms, only recently have researchers been able to capture, store, and analyze large amounts of proteomic data. Advances in nano-scale fabrication and imaging methodologies now make it possible to rapidly characterize nearly all of the proteins in a cell sample with single-molecule precision. With these advancements bolstering us, we’re ready for a proteomics revolution that will finally fuel precision medicine and lead to safer, more effective drugs.
Proteomics and drug development – new solutions to well-known problems
As our ability to quantify, localize, and study the individual proteins making up the proteome advances, biological research will change in ways we can’t imagine. Nonetheless, we expect the expanded use of proteomics will positively impact drug development in some essential and predictable ways. In this post, we cover some of the ways next-generation proteomics technologies can improve our understanding of the mechanisms of disease. In the next post in this series, we’ll cover how next-generation proteomics can help researchers identify potential drug targets and make effective drugs with few side effects.
Improving our understanding of the mechanisms of disease
Genomics can identify genetic variants associated with disease. However, simply knowing that a variant is associated with a disease tells us little about if or how the variant causes disease.
Instead, we need to know how genetic variants impact proteins – the molecular machines behind cellular functions. It is the activities of proteins that usually cause cells to be healthy or diseased. Genetic variants can alter proteins in many ways (Figure 1). For example, they might:
- Alter a protein’s structure and function
- Keep the protein from functioning at all
- Cause the protein to interact with other parts of the cell in non-productive ways
- Change where the protein localizes in the cell
- Change where the protein is produced in the body
- Change the protein’s abundance
- A genetic variant might just be a bystander. It might have no mechanistic relevance at all.
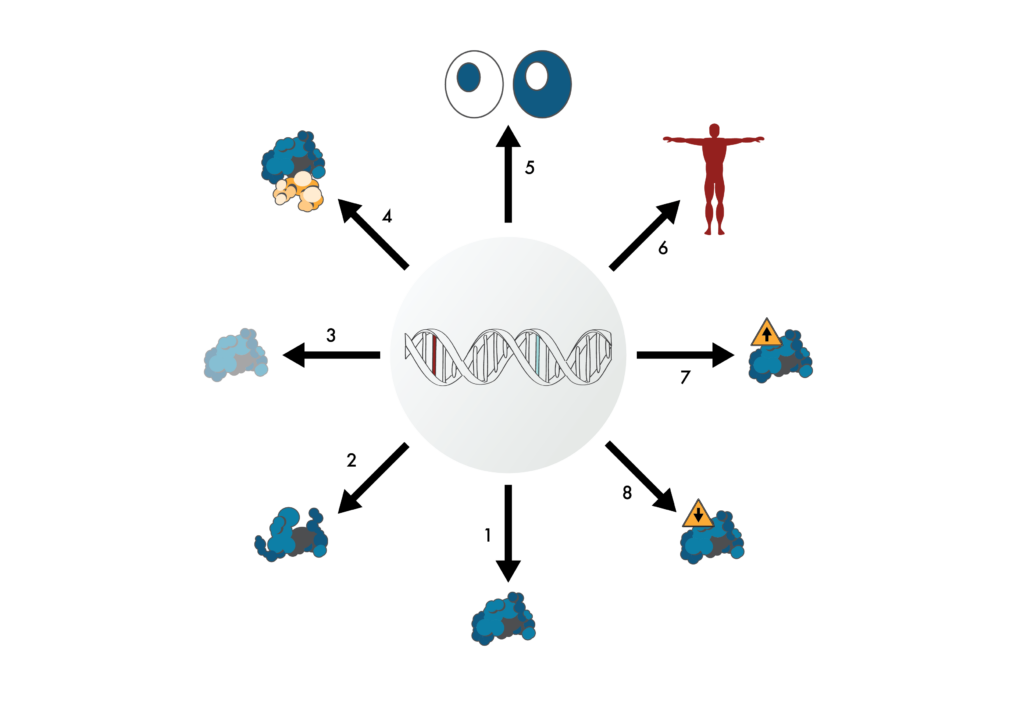
Using proteomics to understand the mechanisms of disease.
Although genomics data and genetic variants (red and blue base pairs) can point researchers to proteins that may be involved in disease, they provide little information about how those variants lead to disease. Genetic variants might (1) not have any impact on the encoded proteins, (2) encode proteins with altered structure, (3) with loss of function, (4) with non-productive interactions, (5) with different sub-cellular localization, (6) with expression in a different part of the body, (7) with increased expression, or (8) with decreased expression. Proteomics data can help researchers understand how genetic variants lead to disease by indicating which of the mechanisms shown here are most likely. With this knowledge, they can later create drugs that counteract the mechanisms of disease more directly.
Thus, researchers need more than genetic information to understand the mechanisms of disease. Using proteomics, researchers can probe how a genetic variant changes an encoded protein and the cell at large.
For example, let’s say a genetic variant encodes a broken protein that normally increases the abundance of other proteins. Breaking this protein decreases the production of the “downstream” proteins. Researchers can observe this decrease using proteomics. If they know the functions of the downstream proteins, they can hypothesize reasons why changing their abundance leads to disease. Further experiments will solidify the causal links to disease.
In addition, many diseases have no genetic origins. Their causes instead lie in interactions between cells and the environment. These include interactions with:
- Disease-causing organisms (pathogens)
- Toxic chemicals
- Allergens
- Lifestyle factors
Genomics cannot give us clues about the causes of these diseases, but proteomics can. For example, in some diseases, proteins glom onto one another and form “protein aggregates.” These aggregates can disrupt cellular functions and lead to such things as neurological disorders. Often, the forces causing protein aggregation aren’t genetically determined. Thus we cannot observe protein aggregation with genomics but we can see it with proteomics. When developing drugs to treat aggregates, we can also use proteomics to quickly determine whether the aggregates disappear.
Check out the next post in this series to learn how these insights can contribute to the development of more effective drugs.
This blog post is an excerpt from the our white paper titled,“Proteomics and the development or precision medicines against cancer.” Download the full white paper here.
MORE ARTICLES

