Proteomics and the development of precision medicines against cancer (Part 3)

Tyler Ford
February 28, 2023
Cancer is a heterogenous mixture of diseases characterized by, among other things, the abnormal, uncontrolled growth of cells derived from otherwise healthy tissues (Hanahan 2022). Although cancer cells sometimes grow into balls of cells and stop there (so-called benign tumors), often they gain the ability to disperse throughout the body, seed the growth of other tumors, disrupt the function of a variety of organs, and ultimately kill patients. In this series of blog posts, we discuss how proteomics can drive the creation and use of “precision medicines” that target the processes enabling cancer growth and stop cancer in its tracks.
Want to read the full series now?
The proteome and cancer treatment
When it comes to developing new cancer treatments, proteomics will accelerate research in a number of ways. These include:
- Prioritizing which mutant genes to target based on the abundance of the encoded proteins. Generally it is better to target proteins produced at a higher level in cancer cells as opposed to healthy cells. This can make drugs more selectively active on cancer cells and thereby decrease side effects and toxicity.
- Identifying potential targets downstream of a driver mutation. The protein encoded in a mutated gene may be difficult to target with a drug for a variety of reasons. By inserting this mutation into healthy cells and assessing how other protein levels change as a result, researchers can identify additional therapeutic targets. E.g. proteins whose production increases after the mutated gene is added to healthy cells and which have a known association with growth signaling might make good drug targets.
- Mining broad changes in post-translational modification and protein production. If proteins have different post-translational modifications in cancer cells or are produced at drastically different levels in cancer cells compared to healthy cells, they may be involved in the molecular mechanisms driving the cancer. Researchers can thus design drugs to alter these post-translational modifications or change the production of the aberrant proteins to determine if cancer cell growth is affected. If so, drugs targeting these modifications or proteins can be prioritized for testing in patients.
Once a therapeutic target has been established and validated through the clinical trial process, proteomics can continue to help doctors achieve more effective treatments. Proteomics can help doctors identify patients in which treatments are working well, in which cancer cells appear to be developing drug resistance, and in which treatments are failing.
To understand how this is useful, it’s informative to look at one of the most effective precision medicines developed to date, the anti-cancer drug, Imatinib (Hochhaus 2017). This drug was developed to treat a cancer of the immune system called “Chronic Myeloid Leukemia” or CML. As discussed in the first post in this series, CML manifests when a translocation between 2 chromosomes creates a fusion of the genes bcr and abl. The BCR-ABL fusion protein encoded in this gene continuously signals for cells to grow and drives cancer progression.
Upon discovering the causal role of BCR-ABL in CML, researchers set out to develop drugs that could inhibit its activity. They searched through a library of chemicals to find compounds that did just that and, after finding a promising hit, optimized this compound to be more easily delivered to the body and to act more selectively on BCR-ABL and not other, similar proteins. Imatinib was the result and, although it takes months for its effects to be achieved, many patients respond incredibly well to this precisely targeted drug (Sacha 2014, Hochhaus et al 2017).
Unfortunately, Imatinib does not work in all patients. Some of them have cancer cells that produce “efflux pump” proteins which pump the drug out before it can have beneficial impacts (Illmer et al 2004). Others have additional mutations in the bcr-abl fusion gene that prevent imatinib from interacting with the fusion protein productively, and still others have mutations that increase production of the BCR-ABL protein (Hochhaus et al 2002, Osman and Deininger 2021).
Researchers have created Imatinib successors that retain their activity in the face of many of these resistance mechanisms. However, many years of additional research were required to elucidate the mechanisms of resistance and optimize these newer drugs. Even so, some of these newer compounds have stronger side-effects than Imatinib and may be risky for some subgroups of CML patients to take (Osman and Deininger 2021). In addition, Imatinib and its successors are only effective during the early, less aggressive stages of CML. If patients advance beyond this stage, drug resistance is common and further progression is often BCR-ABL independent because the cancer cells acquire additional driver mutations. Finally, even when successfully treated, most patients must keep taking Imatinib or one of its successors for life to avoid relapse. This can be very expensive and efforts are ongoing to determine what distinguishes patients who don’t relapse from those who do.
Given the above issues, even in the face of tremendous success, proteomics can accelerate and enhance the development of precision medicines like Imatinib in a number of ways. These include:
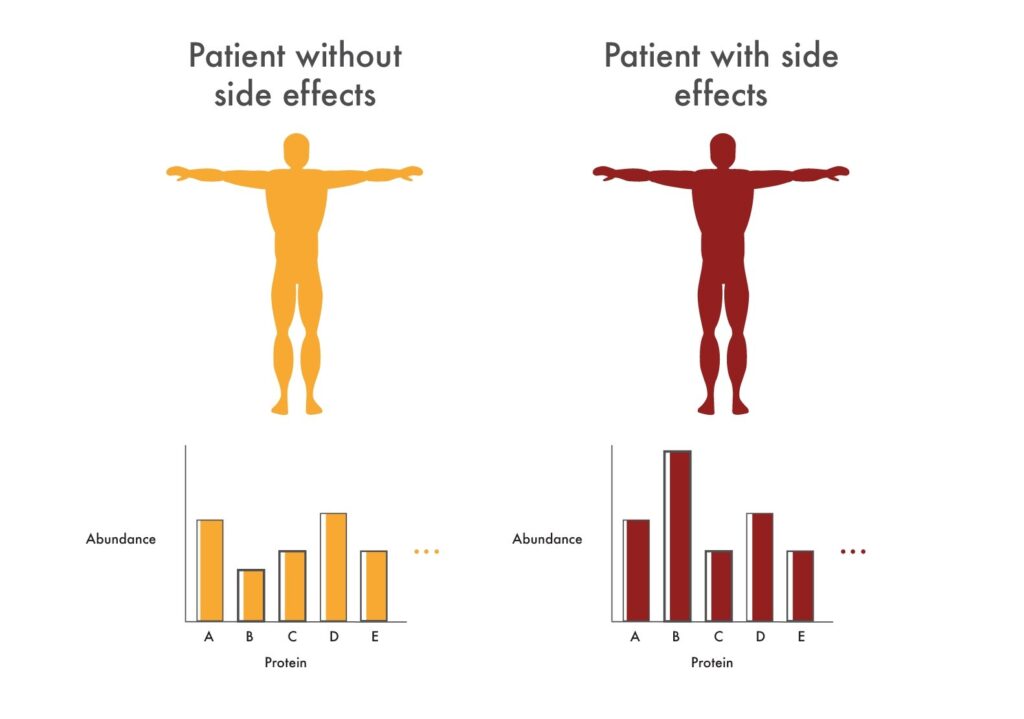
Researchers can use proteomics to look for changes in protein abundance associated with adverse reactions to new cancer drugs. These changes in abundance may indicate that the drugs being tested are not selective enough for the pathway researchers would like to target. For example, they might see drastic changes to proteins involved in heart health. These could indicate that the tested drugs will cause cardiac issues and researchers could make efforts to alter the drugs so they impact these proteins less.
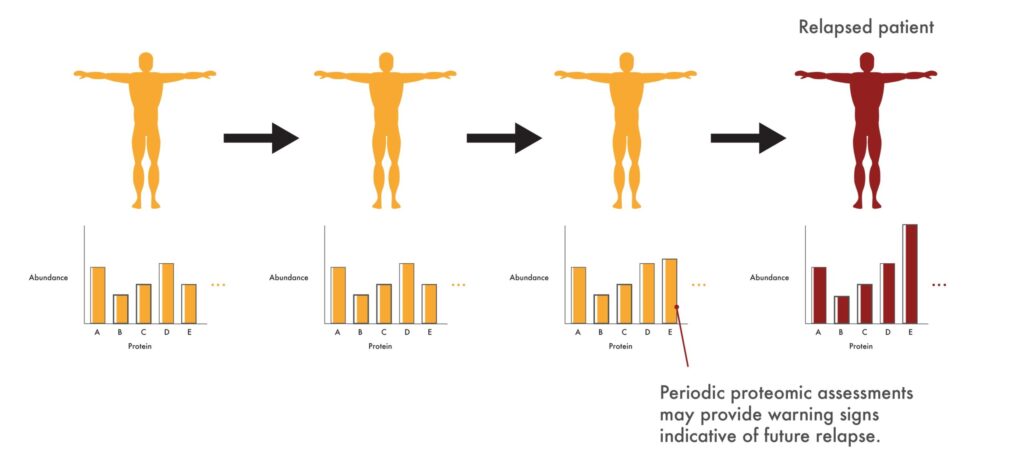
In the clinic, proteomics may give physicians early warning signs that a patient’s cancer is developing resistance to a drug like Imatinib. They might, for instance, see an increase in proteins associated with cancer cell growth. As a result, they could take swift action to get such patients on a second line drug before the cancer returns in full force and starts having strong impacts on patient health.
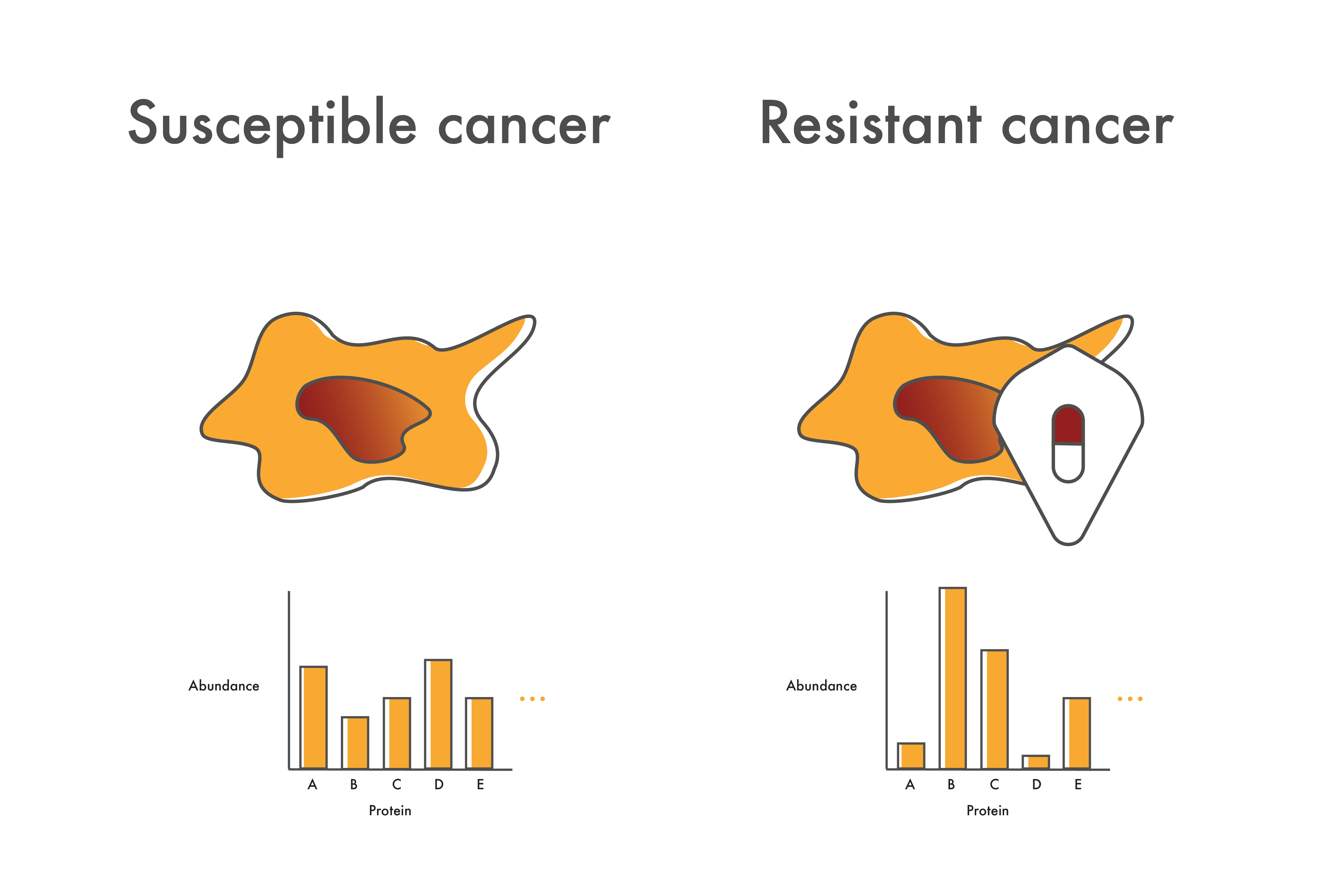
In patients who do develop resistance, cancer cell proteome measurements could provide clues as to the cause of resistance. Even if they don’t know what mutations are involved, researchers might see increased levels of driver proteins like BCR-ABL or increases in other proteins like the efflux proteins discussed above. With this information in hand, researchers could direct additional studies to investigate these possible sources of resistance and accelerate the development of alternative therapeutics.
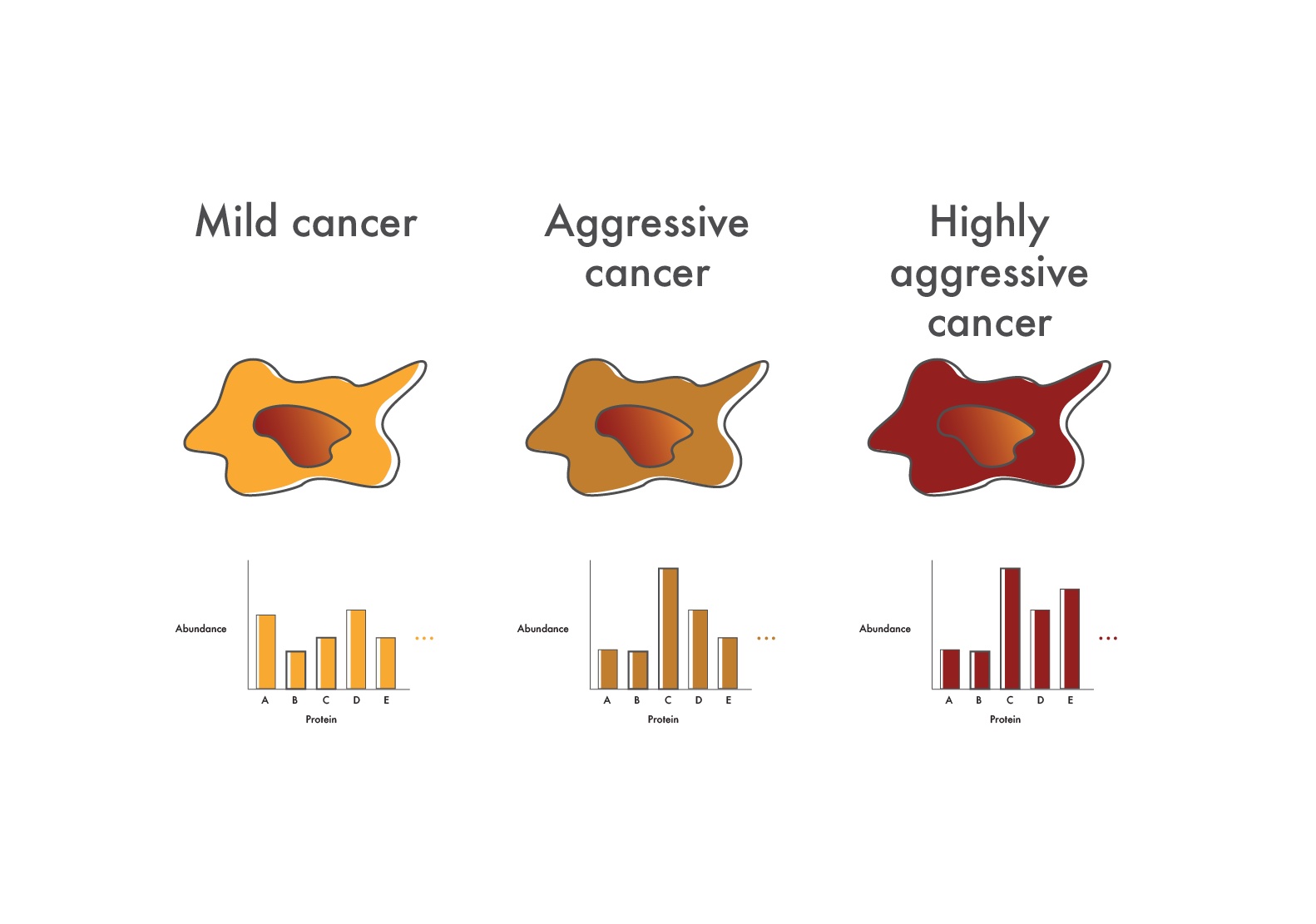
In cancers like CML where advanced disease is refractory to precisely targeted drugs, researchers can use proteomics to determine what additional pathways are up-regulated in the more aggressive cancer cells. They might, for instance, look for increased phosphorylation of signaling proteins and, if they find an increase, take steps to inhibit the signaling proteins involved to stop cancer progression.
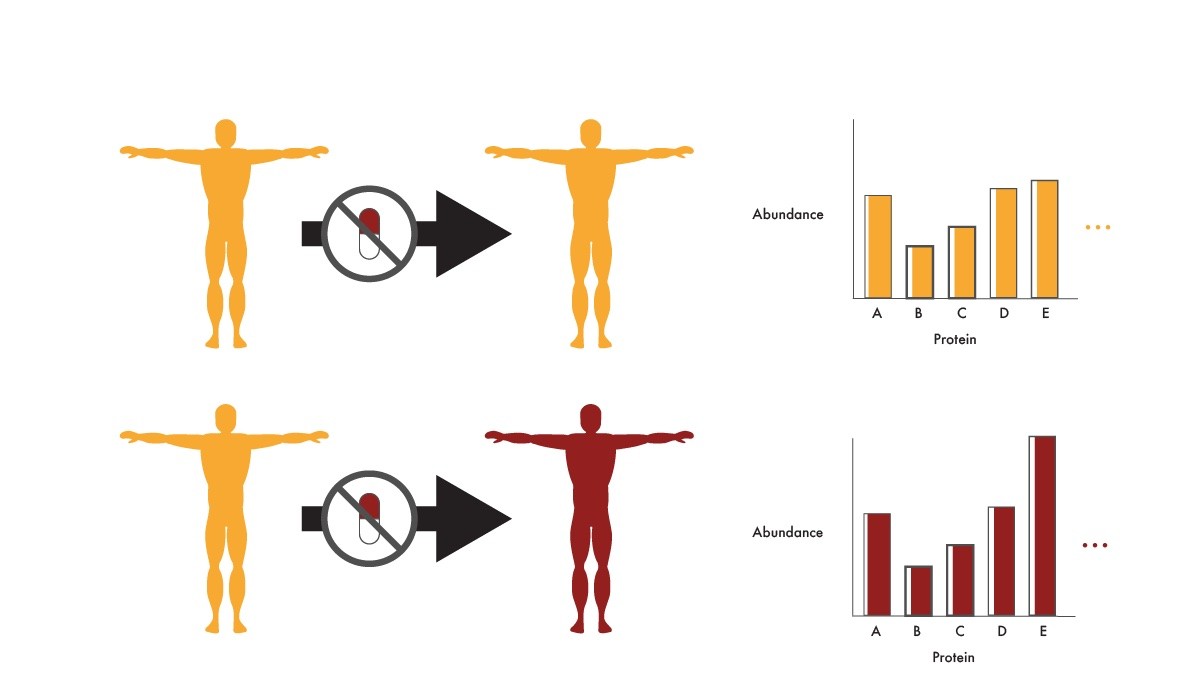
Researchers can use proteomics to look for differences in protein abundance between patients who stay in remission after they are taken off a precision medicine and those who relapse. By associating particular protein abundances with relapse, physicians could stratify patients who are good or bad candidates for being taken off the precision medicine. They could also develop new treatments that increase the production or activity of proteins associated with remission after ending therapy. These efforts could get patients off of the anti-cancer drugs faster with less risk of relapse. This would lower expenses and prevent patients from experiencing the side effects associated with these drugs.
Proteomics and cancer – a healthier outlook for the future
Proteomics should make it possible to more quickly identify the molecular causes of various types of cancer, stratify patients by disease severity to prioritize treatment, and improve clinical outcomes. As physicians and researchers use advanced proteomics technologies to catalog the proteomes of more and more cancers, they’ll be able to create new protein-based diagnostic tools, identify molecular targets for therapeutics, understand mechanisms of drug resistance, and monitor therapeutic effectiveness. With the wide availability of proteomics data, there’s a much brighter future in store for many cancer patients.
MORE ARTICLES