Proteoforms and EGFR signaling – Applications of proteomics in cancer

Tyler Ford
May 15, 2025
It’s well-understood that post-translational modifications influence the activity of cell signaling pathways, which control critical processes like proliferation, migration, and differentiation. Less appreciated is how distinct combinations of modifications or modification patterns on individual protein variants (proteoforms) alter signaling and shape phenotypic outcomes. That’s why studying proteoforms is essential.
The cancer research literature offers many examples suggesting the importance of proteoforms, and studies of epidermal growth factor receptor (EGFR) provide some notable cases. One such case is Huang et al., 2010, led by researchers in Forest White’s lab at MIT. Their findings—and those of related studies—suggest modification patterns on EGFR proteoforms may significantly influence signaling outcomes.
Learn how we quantify proteoforms on the NautilusTM Proteome Analysis Platform
A brief introduction to the epidermal growth factor receptor (EGFR)
EGFR regulates diverse cellular processes and is frequently mutated in cancer. Its signaling is often proliferative but highly nuanced. The receptor consists of:
- An extracellular domain that binds epidermal growth factor (EGF)
- A transmembrane domain
- An intracellular domain with tyrosine kinase activity and phosphorylatable tyrosines
EGF binding induces EGFR dimerization, triggers autophosphorylation of tyrosines on EGFR, and enables phosphorylation of downstream proteins. EGFR phosphotyrosines can serve as docking points for effectors that amplify, modulate, or terminate signaling (See Lemmon and Schlessinger 2010 and Halder et al., 2023 for reviews focused on receptor tyrosine kinases and EGFR).
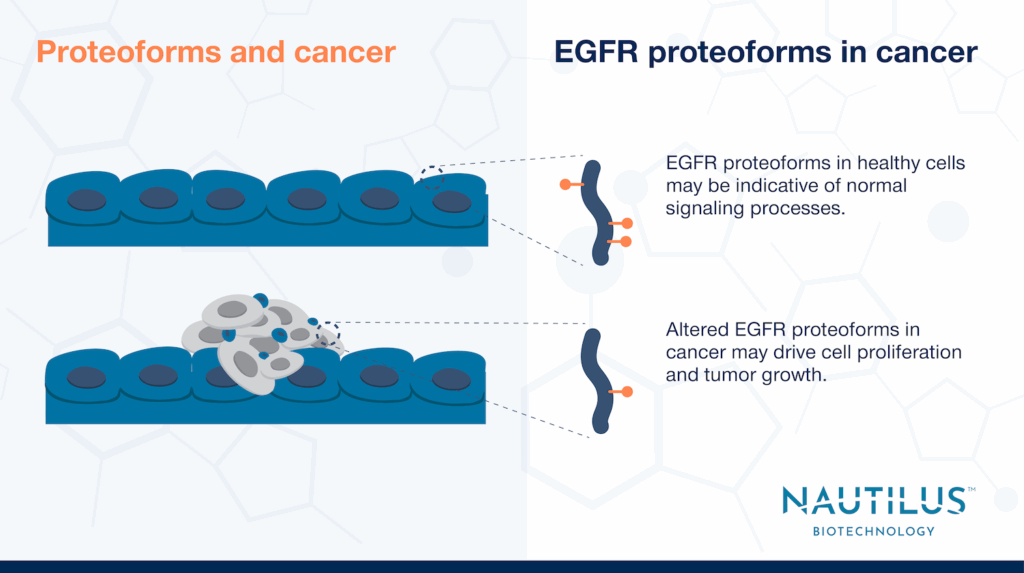
The importance of proteoforms in EGFR signaling
EGFR signaling isn’t linear and is very complex, but is often implicated in cancer. The White Lab specifically investigated a cancer-associated, constitutively active mutant of EGFR called EGFRvIII. It cannot bind EGF due to a deletion in its extracellular domain, is often detected in glioblastoma, and has greater oncogenic potential than its wildtype counterpart (See review by Guo et al., 2015). Huang et al., 2010’s results suggest that changes in EGFRvIII’s potential for combinatorial modification may modulate its impacts on cell growth.
To explore how EGFRvIII tyrosines affect signaling, the authors mutated four of them to phenylalanine:
- Y845
- Y1068
- Y1148
- Y1173
They tested single mutations and these combinations:
- Y1068F, Y1173F
- Y1068F, Y1148F, Y1173F
All single mutations except Y1173F decreased phosphorylation of other EGFR tyrosines. Y1173F increased phosphorylation and only after being combined with Y1068F AND Y1148F was this increase abrogated. Phosphorylation of downstream signaling proteins followed similar trends.
Interestingly all mutations except Y1068F alone decreased cell proliferation. This shows there’s not a 1 to 1 correlation between phosphorylation and proliferation – it depends on the sites and the context.
These results do not necessarily show that single EGFR molecules are modified at multiple sites. However, they suggest that the potential for combinatorial modifications may shape signaling behavior. These results also indicate there is crosstalk between modification sites.
Further work by Huang et al., 2010 showed extracellular signal-regulated kinase (ERK) signaling played a role in the observed growth impacts. ERK1 and 2 are serine-threonine kinases involved in a variety of signaling pathways regulating differentiation, cell migration, proliferation, and more (See Timofeev et al., 2024 for a review on “ERK pathway agonism for cancer therapy”). In this work, ERK phosphorylation was anticorrelated with proliferation and increased in all mutants except the Y1068F single mutant. Subsequent studies have expanded on these results and demonstrated connections between EGFR signaling and other processes. Examples include:
- ERK phosphorylates EGFR threonine 669 and this coincides with downregulation of constitutive EGFR tyrosine phosphorylation. Inhibiting ERK or mutating EGFR T669 prevents this downregulation (Sato et al., 2013).
- EGFR phosphorylation at Y1068 or Y1086 and Y1045 results in EGFR ubiquitination which may shut off EGFR signaling (Sigismund et al., 2013).
Opportunities for targeted proteoform studies focused on EGFR
Together the results above reinforce the idea that patterns of phosphorylation across EGFR molecules may shape signaling and its outcomes. These and many other studies like them suggest we may gain a much better understanding of signaling by studying proteoforms.
By measuring EGFR proteoforms in particular, we may gain clarity in terms of how the EGFR pathway interacts with other pathways and contributes to important processes like proliferation. Understanding how mixtures of EGFR proteoforms impact biology may be the key to developing.
Learn more about the NautilusTM Proteome Analysis Platform.
Learn how next-generation proteomics platforms may impact cancer research.
MORE ARTICLES
